Signaling in heart failure
 Congestive heart failure (CHF) is a worldwide epidemic. It is estimated, for example, that in Europe around 10 million people are suffering from this disease. Despite some progress in medical treatment within the last 10 years, morbidity and mortality of CHF are still high: 70-80% of patients suffering from heart failure will die within the next 8 years. Heart failure develops mainly after myocardial infarction, chronic arterial hypertension, diseases of the cardiac valves (e.g. aortic valve stenosis), viral myocarditis or genetic disease. Associated with all these different disease entities is a profound alteration of the ventricular shape and function, which is triggered by cardiac overload as well as local and systemic activation of specific cytokines and growth factors. As a final common pathway of almost every heart disease leading to heart failure, the left cardiac ventricle dramatically dilates, while the left ventricular walls become increasingly thinner. This progressive dilation markedly increases wall stress, which in turn leads to further damage to the myocardium and diminishes the capability of the heart to pump blood into the circulation.
Our lab study the molecular mechanisms responsible for cardiac hypertrophy and remodeling during heart failure. We showed that the Extracellular Signal-Regulated Kinase (ERK) pathway acts to promote a compensated hypertrophic response, with enhanced contractile function and reduced fibrosis. The activation of this pathway may be a therapeutic strategy in heart failure to preserve contractile function when the pressure overload cannot be easily alleviated.
Congestive heart failure (CHF) is a worldwide epidemic. It is estimated, for example, that in Europe around 10 million people are suffering from this disease. Despite some progress in medical treatment within the last 10 years, morbidity and mortality of CHF are still high: 70-80% of patients suffering from heart failure will die within the next 8 years. Heart failure develops mainly after myocardial infarction, chronic arterial hypertension, diseases of the cardiac valves (e.g. aortic valve stenosis), viral myocarditis or genetic disease. Associated with all these different disease entities is a profound alteration of the ventricular shape and function, which is triggered by cardiac overload as well as local and systemic activation of specific cytokines and growth factors. As a final common pathway of almost every heart disease leading to heart failure, the left cardiac ventricle dramatically dilates, while the left ventricular walls become increasingly thinner. This progressive dilation markedly increases wall stress, which in turn leads to further damage to the myocardium and diminishes the capability of the heart to pump blood into the circulation.
Our lab study the molecular mechanisms responsible for cardiac hypertrophy and remodeling during heart failure. We showed that the Extracellular Signal-Regulated Kinase (ERK) pathway acts to promote a compensated hypertrophic response, with enhanced contractile function and reduced fibrosis. The activation of this pathway may be a therapeutic strategy in heart failure to preserve contractile function when the pressure overload cannot be easily alleviated.
Paper by Michael Mutlak MD:
 Mutlak M, Schlesinger-Laufer M, Haas T, Shofti R, Ballan N, Lewis YE, Zuler M, Zohar Y, Caspi LH, Kehat I. Extracellular signal-regulated kinase (ERK) activation preserves cardiac function in pressure overload induced hypertrophy. Int J Cardiol. 2018 May 24
https://www.ncbi.nlm.nih.gov/pubmed/29857938
Mutlak M, Schlesinger-Laufer M, Haas T, Shofti R, Ballan N, Lewis YE, Zuler M, Zohar Y, Caspi LH, Kehat I. Extracellular signal-regulated kinase (ERK) activation preserves cardiac function in pressure overload induced hypertrophy. Int J Cardiol. 2018 May 24
https://www.ncbi.nlm.nih.gov/pubmed/29857938
Genome organization and gene expression in cardiac remodeling
 Transcriptional regulation in mammals was simplistically viewed as a process in which soluble protein factors are recruited to promoters, located immediately 5′ of a protein encoding gene, to activate or repress transcription. However, more recent data suggest that the nucleus is an ordered three-dimensional organelle, in which the organization of the genome appears to have implications for orchestrated gene expression. Data suggest that the expression of some genes correlates with their genomic environment. This environment consists of interactions of DNA loci with nuclear structural components such as the nuclear lamina and the nucleoporins and interaction of DNA loci with remote sequences on the same or other chromosomes.
Transcriptional regulation in mammals was simplistically viewed as a process in which soluble protein factors are recruited to promoters, located immediately 5′ of a protein encoding gene, to activate or repress transcription. However, more recent data suggest that the nucleus is an ordered three-dimensional organelle, in which the organization of the genome appears to have implications for orchestrated gene expression. Data suggest that the expression of some genes correlates with their genomic environment. This environment consists of interactions of DNA loci with nuclear structural components such as the nuclear lamina and the nucleoporins and interaction of DNA loci with remote sequences on the same or other chromosomes.
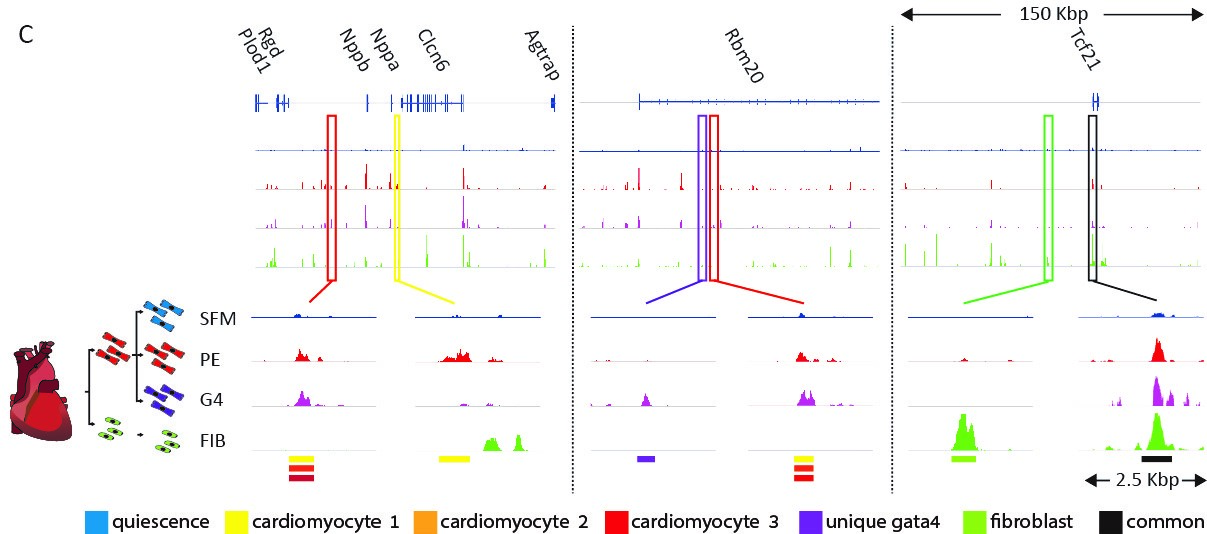 We are trying to understand how cell-type-specific gene expression is controlled by the genome and chromatin in the heart. We performed a genome-wide analysis of regulatory elements (enhancers) in cardiomyocytes and in cardiac fibroblasts using ATAC-seq, H3K27ac ChIP-seq, RNA-seq, and computational transcription factor binding analysis. These analyses enabled us to map the cell-type-specific active enhancers in cardiac fibroblasts and cardiomyocytes and outline the transcription factor families that control them. Our studies showed that in both cell types, distal enhancers, containing concentrated combinatorial clusters of multiple tissue expressed transcription factor recognition motifs, are combinatorically clustered around tissue specific genes.
We are trying to understand how cell-type-specific gene expression is controlled by the genome and chromatin in the heart. We performed a genome-wide analysis of regulatory elements (enhancers) in cardiomyocytes and in cardiac fibroblasts using ATAC-seq, H3K27ac ChIP-seq, RNA-seq, and computational transcription factor binding analysis. These analyses enabled us to map the cell-type-specific active enhancers in cardiac fibroblasts and cardiomyocytes and outline the transcription factor families that control them. Our studies showed that in both cell types, distal enhancers, containing concentrated combinatorial clusters of multiple tissue expressed transcription factor recognition motifs, are combinatorically clustered around tissue specific genes.
Paper be Tal Golan-Lagziel (MD/PhD candidate):

Golan-Lagziel T, Lewis YE, Shkedi O, Douvdevany G, Caspi LH, Kehat I. Analysis of rat cardiac myocytes and fibroblasts identifies combinatorial enhancer organization and transcription factor families. J Mol Cell Cardiol. 2018 Mar;116:91-105.
https://www.ncbi.nlm.nih.gov/pubmed/?term=kehat+and+golan-lagziel https://www.sciencedirect.com/science/article/pii/S0022282818300373?via%3DihubGrowth and maintenance of the sarcomere
 Heart failure is a global problem with an estimated prevalence of 38 million patients worldwide, a number that is increasing with the ageing of the population. Survival after a diagnosis of heart failure has improved during the past 30 years; however, despite the modest improvement, the 5-year mortality is still approximately 50% - worse than that of many cancers. Efforts to enhance the understanding of the pathobiology of heart failure and to develop new approaches for treatments are direly needed.
Heart failure is a global problem with an estimated prevalence of 38 million patients worldwide, a number that is increasing with the ageing of the population. Survival after a diagnosis of heart failure has improved during the past 30 years; however, despite the modest improvement, the 5-year mortality is still approximately 50% - worse than that of many cancers. Efforts to enhance the understanding of the pathobiology of heart failure and to develop new approaches for treatments are direly needed.
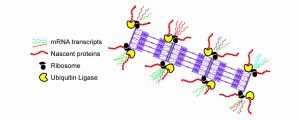
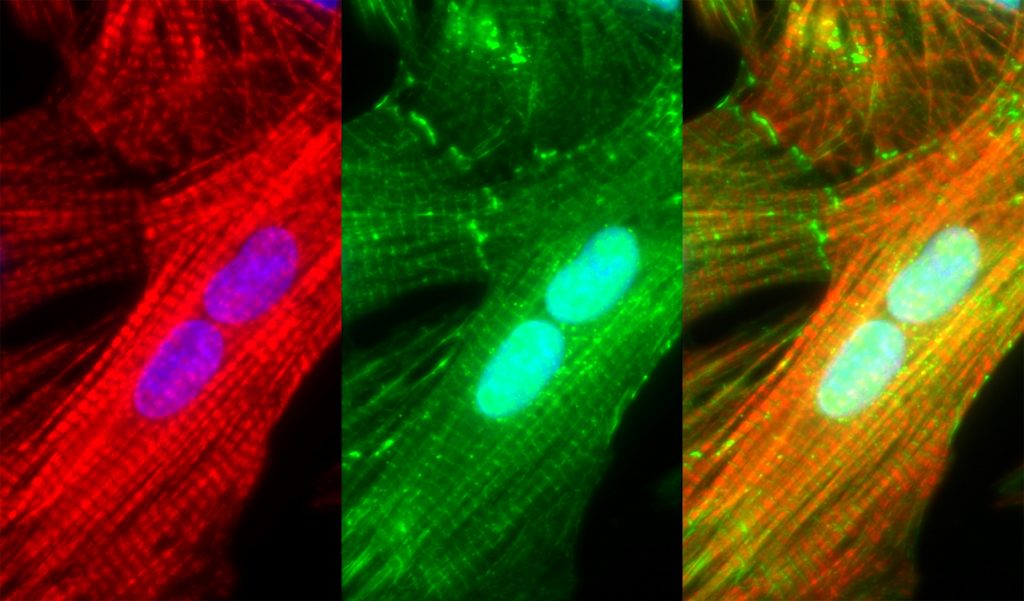 Sarcomeric dysfunction plays a central role in the reduced cardiac pump function in heart failure. We study the maintenance of the sarcomere, the basic contractile macromolecular complex of cardiomyocytes. We performed single-cell analysis of cardiomyocytes using imaging of mRNA and protein synthesis and demonstrate that three distinct mechanisms are responsible for the maintenance of the sarcomere: mRNAs encoding for sarcomeric proteins are localized to the sarcomere, ribosomes are localized to the sarcomere with localized sarcomeric protein translation, and finally, a localized E3 ubiquitin ligase allow efficient degradation of excess unincorporated sarcomeric proteins. We showed that these mechanisms are distinct, required, and work in unison, to ensure both spatial localization, and to overcome the large variability in transcription. These studies provides a novel understanding of the sarcomere maintenance mechanisms.
Sarcomeric dysfunction plays a central role in the reduced cardiac pump function in heart failure. We study the maintenance of the sarcomere, the basic contractile macromolecular complex of cardiomyocytes. We performed single-cell analysis of cardiomyocytes using imaging of mRNA and protein synthesis and demonstrate that three distinct mechanisms are responsible for the maintenance of the sarcomere: mRNAs encoding for sarcomeric proteins are localized to the sarcomere, ribosomes are localized to the sarcomere with localized sarcomeric protein translation, and finally, a localized E3 ubiquitin ligase allow efficient degradation of excess unincorporated sarcomeric proteins. We showed that these mechanisms are distinct, required, and work in unison, to ensure both spatial localization, and to overcome the large variability in transcription. These studies provides a novel understanding of the sarcomere maintenance mechanisms.
Paper by Yair Lewis MD:
 Lewis YE, Moskovitz A, Mutlak M, Heineke J, Caspi LH, Kehat I. Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance. J Mol Cell Cardiol. 2018 Mar;116:16-28.
https://www.ncbi.nlm.nih.gov/pubmed/29371135
https://www.sciencedirect.com/science/article/pii/S002228281830021X?via%3Dihub
Lewis YE, Moskovitz A, Mutlak M, Heineke J, Caspi LH, Kehat I. Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance. J Mol Cell Cardiol. 2018 Mar;116:16-28.
https://www.ncbi.nlm.nih.gov/pubmed/29371135
https://www.sciencedirect.com/science/article/pii/S002228281830021X?via%3DihubVascular and valve calcification
 Most aging individuals have progressively enlarging accumulation of calcium in their major arteries. These vascular calcifications, that appear in the walls of blood vessels such as the aorta, coronary, carotid and femoral arteries and in the leaflets of heart valves cause stiffness and impair hemodynamics resulting in hypertension and organ ischemia, valve stenosis, cardiac hypertrophy, and congestive heart failure. The presence of such calcification in any arterial wall is associated with a 3–4-fold higher risk for mortality and cardiovascular events. We are trying to elucidate the molecular mechanisms responsible for vascular and valve calcifications and find novel approaches to stop this devastating process.
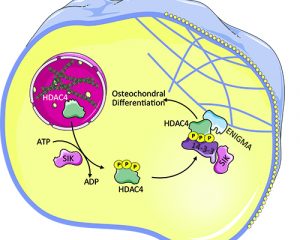
We showed that HDAC4 is a positive regulator driving this pathology. HDAC4 can shuttle between the nucleus and cytoplasm, but the cytoplasmic rather than the nuclear activity of HDAC4 promotes calcification. The cytoplasmic location and function of HDAC4 is controlled by the activity of salt-inducible kinase (SIK). In the cytoplasm, HDAC4 binds and its activity depends on the adaptor protein ENIGMA (Pdlim7) to promote vascular calcification. These results establish a cytoplasmic role for HDAC4 and identify HDAC4, SIK, and ENIGMA as mediators of vascular calcification.
Most aging individuals have progressively enlarging accumulation of calcium in their major arteries. These vascular calcifications, that appear in the walls of blood vessels such as the aorta, coronary, carotid and femoral arteries and in the leaflets of heart valves cause stiffness and impair hemodynamics resulting in hypertension and organ ischemia, valve stenosis, cardiac hypertrophy, and congestive heart failure. The presence of such calcification in any arterial wall is associated with a 3–4-fold higher risk for mortality and cardiovascular events. We are trying to elucidate the molecular mechanisms responsible for vascular and valve calcifications and find novel approaches to stop this devastating process.
We showed that HDAC4 is a positive regulator driving this pathology. HDAC4 can shuttle between the nucleus and cytoplasm, but the cytoplasmic rather than the nuclear activity of HDAC4 promotes calcification. The cytoplasmic location and function of HDAC4 is controlled by the activity of salt-inducible kinase (SIK). In the cytoplasm, HDAC4 binds and its activity depends on the adaptor protein ENIGMA (Pdlim7) to promote vascular calcification. These results establish a cytoplasmic role for HDAC4 and identify HDAC4, SIK, and ENIGMA as mediators of vascular calcification.
Paper by Alon Abend (MD/PhD candidate):
 Abend A, Shkedi O, Fertouk M, Caspi LH, Kehat I. Salt-inducible kinase induces cytoplasmic histone deacetylase 4 to promote vascular calcification. EMBO Rep. 2017 Jul;18(7):1166-1185
https://www.ncbi.nlm.nih.gov/pubmed/28588072
http://embor.embopress.org/content/18/7/1166.long
Abend A, Shkedi O, Fertouk M, Caspi LH, Kehat I. Salt-inducible kinase induces cytoplasmic histone deacetylase 4 to promote vascular calcification. EMBO Rep. 2017 Jul;18(7):1166-1185
https://www.ncbi.nlm.nih.gov/pubmed/28588072
http://embor.embopress.org/content/18/7/1166.long