Heart failure is a global problem with an estimated prevalence of 38 million patients worldwide, a number that is increasing with the ageing of the population. Survival after a diagnosis of heart failure has improved during the past 30 years; however, despite the modest improvement, the 5-year mortality is still approximately 50% – worse than that of many cancers. Efforts to enhance the understanding of the pathobiology of heart failure and to develop new approaches for treatments are direly needed.
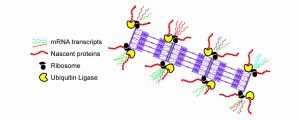
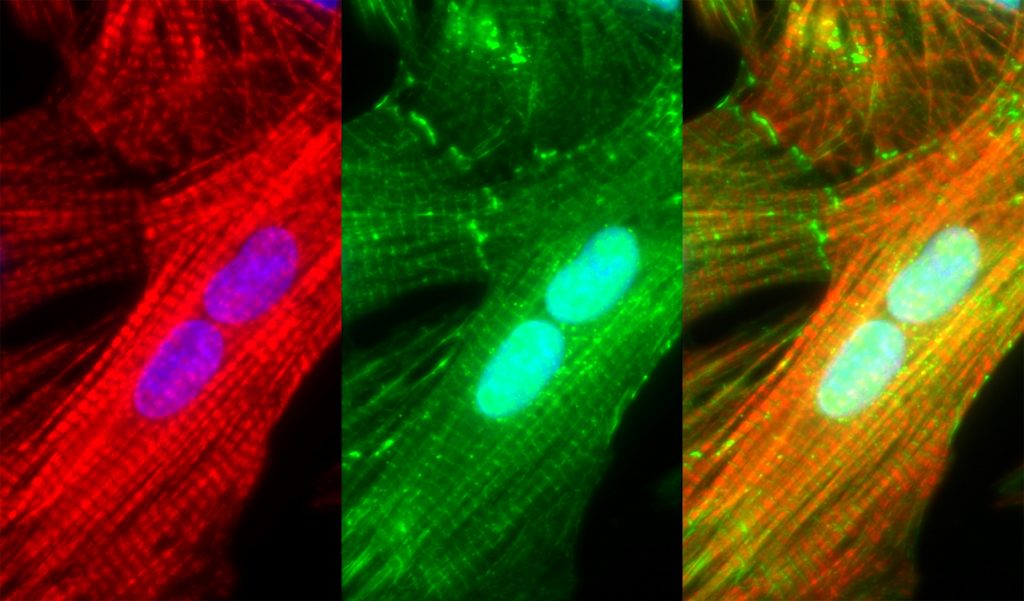
Sarcomeric dysfunction plays a central role in the reduced cardiac pump function in heart failure. We study the maintenance of the sarcomere, the basic contractile macromolecular complex of cardiomyocytes. We performed single-cell analysis of cardiomyocytes using imaging of mRNA and protein synthesis and demonstrate that three distinct mechanisms are responsible for the maintenance of the sarcomere: mRNAs encoding for sarcomeric proteins are localized to the sarcomere, ribosomes are localized to the sarcomere with localized sarcomeric protein translation, and finally, a localized E3 ubiquitin ligase allow efficient degradation of excess unincorporated sarcomeric proteins. We showed that these mechanisms are distinct, required, and work in unison, to ensure both spatial localization, and to overcome the large variability in transcription. These studies provides a novel understanding of the sarcomere maintenance mechanisms.
Paper by Yair Lewis MD:
Lewis YE, Moskovitz A, Mutlak M, Heineke J, Caspi LH, Kehat I. Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance. J Mol Cell Cardiol. 2018 Mar;116:16-28.
https://www.ncbi.nlm.nih.gov/pubmed/29371135
https://www.sciencedirect.com/science/article/pii/S002228281830021X?via%3Dihub